Search Products
Metal
TRIVALENT CHROMIUM PLATING EXPERIMENTS REPORT
REPORTS OF TRIVALENT CHROMIUM PLATING EXPERIMENTS ON NICKEL DEPOSIT
INTRODUCTION
During many years until now, chrome plating have been doing on the basic solution of hexavalent chromium – chromic acid (CrO3), sulfuric acid and some additives. This kind of solution has some advantages like stable operation, economic, high tolerance of contamination….How ever, it also has some disadvantages like containing hexavalent chromium (Cr6+), this is a strong oxidizer in the natural environment, causes bad effects to working people, corrodes the equipment…so it’s very dangerous for the environment if the waste water is not treated properly.
Because of those disadvantanges, there are many formulas of chromium plating based on trivalent chrome are established, and there are many commercial formulas in the world. In Europe, many chrome plated parts must be done in trivalent chromium.
In Vietnam, even the Government has not set up any regulation on trivalent or hexavalent chromium plating, but because of the commercial relationship between Vietnam and many other countries, particularly with Europe, Japan and America so the application of trivalent chromium plating is needed in near future. For that reason, the testing some trivalent chromium plating formula then selecting a suitable one to apply in end users is a highly needed in the plating industry.
CONTENT
Since May, 2010, MDI Metal has been testing a trivalent chromium plating solution on bright nickel deposit with process as following:
1, Degreasing
2, Alkaline Copper Strike
3, Semi-Bright Nickel
4, Bright Nickel
5, Trivalent Chromium plating:
- Proprietary trivalent chromium solution: 500 lít
- A DC rectifier: 500A – 15V
- Electric heater (needed when making up the solution).
- Air agitation.
- Anode: Carbon Graphite.
- An Ion-exchanger to remove metal contaminants (lead, copper, zinc, nickel, iron…).
The testing process was done on many kinds of products: bicycle parts, motorbike parts, furniture…and we received the following results:
1, The deposit of chrome from Trivalent solution had a light hue of blue, but abit darker to compare with the one came out from hexavalent solution.
2, The needed voltage for Trivalent solution was higher 3-6V to compare with Hexavalent solution.
3, The plating solution of Trivalent chromium had low tolerance of metal contaminats (copper, lead, nickel, zinc, iron…) to compare with Hexavalent solution. However, with the help of Ion Exchanger, the contaminants were removed and the Trivalent solution had very stable operation.
4, The deposit from Trivalent solution had very good distribution and covering power, some little holes was chrome plated easily to compare with Hexvalent solution.
5, Can be dis-continuos plating in Trivalent solution, that means if the electric powder was stopped during plating, it can be plated again without burning, white-wash..etc…The deposit was also not “burned” in high current density area.
7, The graphite anode must have the density above 1.8g/cm3 . Other graphites which have lower density are all not suitable, bacause they could be dissolved in the solution and influence to the color of the deposit.
8, The chrome deposit from Trivalent solution had lower corrosion protection to compare with the one from hexavalent solution. However, if the deposit was coated with one layer of anti-corrosion agent, the trivalent chrome would have the same corrosion protection like hexavalent chrome.
9, The plating speed was high, about 0.13 micron/minute.
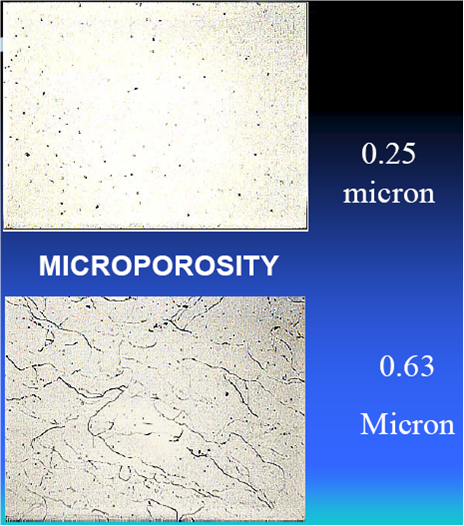
10, The chrome deposit from trivalent solution had micoporous itself when the thickness reaches above 0.5 micron. By this feature, there may be no need to use microporous nickel plating in the nickel plating processes.
11, The treatment of wast water was simple, just one step of neutralization by alkaline agent. The solid waste after treatment also lower to compare with the treatment of hexavalent chrome.
CONCLUSION
The propriatary formula of trivalent chromium plating which MDI Metal has been testing can be applied commercially in many factories with advantages:
1, Safe for the environment because of not using of hexavalent chrome and not using of lead anode.
2, Much lower cost for waste treatment to compare with hexavalent chromium plating.
3, No “burning” in high current density areas.
4, The deposit has microporous itself, so there is no need microporous nickel before chrome plating.
5, The graphite anode has very high long-life, there is nearly no need replacement.
6, High distribution and covering power, so recessive areas can be plated.
7, High tolerance of discontinous plating without the effects to quality of the deposit.
8, Good thickness distribution.